Bioequivalence Testing
OINDP
Nasal Spray Bioequivalence — Where Regulators Are Raising the Bar
The FDA has opened an in vitro–only pathway for complex nasal suspensions, making your dataset the decisive evidence in an ANDA submission.
Most MDRS workflows require multiple overnight runs of ~3,000 particles each, which are then stitched together into a single dataset. This introduces variability and weakens statistical confidence.

At SizeID.bio, we deliver one dataset, one sample:
-
Each analysis captures over 10,000 particles and agglomerates within a single dataset — delivering statistical confidence 10× higher than conventional MDRS workflows, with full traceability and validated reproducibility.
-
Our laboratory workflow acquires 10,000+ particles in a single run, completed in hours rather than overnight. For sponsors, this means faster study completion, earlier bioequivalence readouts, and accelerated submission timelines — turning analytical bottlenecks into competitive advantage.
-
Each measurement includes continuous S/N monitoring with defined positive and negative controls. Quality metrics are recorded throughout acquisition, ensuring USP <858> compliance and verified data integrity in every run.
-
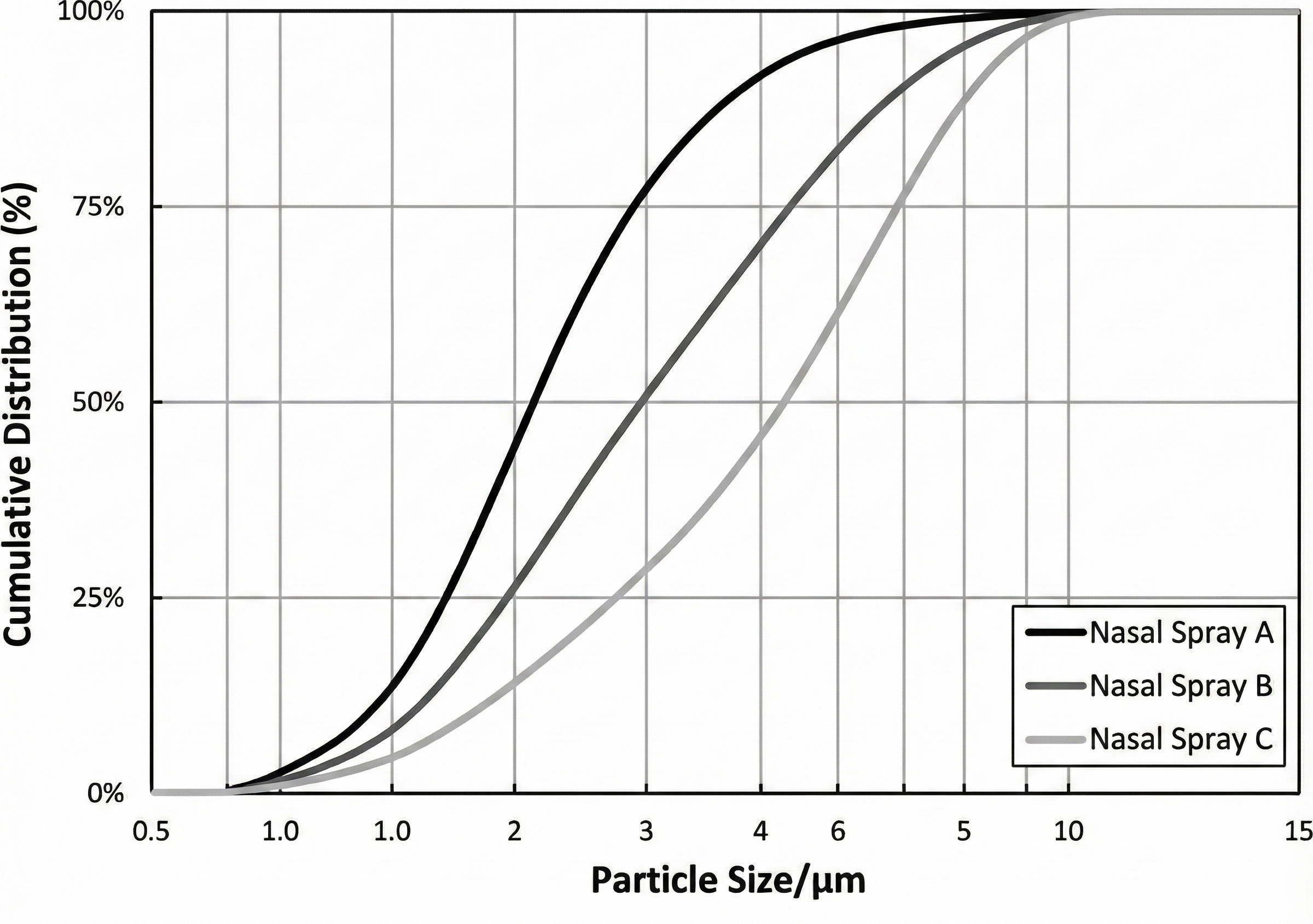
Particle size distribution — especially the proportion of the fine fraction below 5 µm — directly influences drug release kinetics. A higher amount of fine particles accelerates dissolution and shortens the drug-release half-life (T₀.₅), representing a critical quality attribute (CQA) for bioequivalence in OINDPs.
-
Structured for FDA and EMA review, aligned with current PSGs and guideline terminology. Each dataset is fully traceable — from raw spectra to processed PSD results — and includes validation tables, statistical summaries, and audit-ready metadata for transparent, reproducible review.
The logarithmic particle size distribution (1–5 µm) clearly differentiated the three nasal spray formulations. Overall, the log-scale comparison highlighted a clear rank order in particle size: Spray A being the finest, Spray B intermediate, and Spray C the coarsest, with minimal overlap at the distribution tails but distinct differences in their modal regions.
Fluticasone | 3µm | 1s Raman