DeepMorph-Guided MDRS
for bioequivalence, stability,
and contamination control.
Audit-Ready Particle Evidence
At SizeID.bio, we make the smallest differences visible – from OINDP bioequivalence to parenteral stability and contamination control strategy CCS.
Accelerate bioequivalence and CMC decisions with DeepMorph-guided MDRS: high-throughput, particle-level Raman plus imaging AI to generate statistically robust, ingredient-specific particle evidence. Your deliverables are audit-ready and aligned with your GMP expectations.
Why Choose SizeID.bio?
Why Choose SizeID.bio?
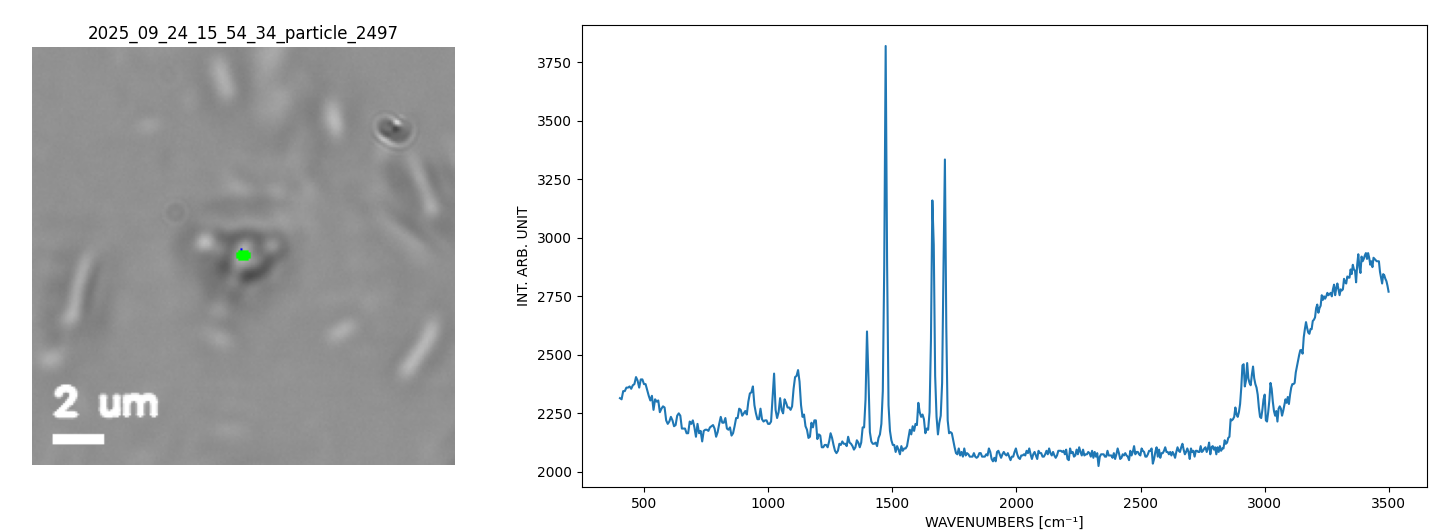
Single 1.7 µm API Particle in a Nasal Spray Suspension (1s Raman).
Typical limitations in the market
-
Many systems struggle to collect usable spectra from small or weakly scattering particles- forcing slow acquisition settings and reducing practical throughput.
-
It’s common to rely on overnight runs that still deliver limited chemical specificity or insufficient particle statistics for confident decisions.
-
Signal quality and “hit” performance are often assessed only before/after the run—so reliability checks require additional controls, rework, or repeat measurements.
-
Below a few microns, blind spots increase: fewer particles are detected, fewer are identified, and size/ID distributions become less representative.
At SizeID.bio, we quantify what others miss: sub-micron particles with AI-directed Raman, built for regulatory decisions.
SizeID.bio advantage
-
When required, we deliver high-count datasets (10,000+ particles) to increase statistical power for in-vitro BE and sensitive comparability questions.
-
Focused contamination investigations designed for root cause, source tracing, trending, and CAPA enablement. So findings translate into decisions, not just datasets.
-
Built-in signal-to-noise verification and in-run performance controls continuously check signal and hit quality. Reducing rework and strengthening confidence in identifications. Typical turnaround is ~1–6 hours for most workflows.
-
Reliable detection down to ~500 nm by combining DeepMorph deep learning (for particle detection and targeting) with Raman identification and integrated spectral algorithms that discriminate closely related materials and sources. Supported by calibration and quality controls.
Your Profit:
For bioequivalence, you need statistical power and drug-specific discrimination.
For parenteral stability, you need to track changes over time | proteinaceous vs. silicone oil vs. extrinsic species.
For contamination control, you need to identify the source, link it to your process, and implement effective CAPA.
With SizeID.bio, you get the depth when you need it | and the precision when it matters.