Data You Can Trust
Streamlined Testing
At SizeID.bio, we make the smallest differences visible – from OINDP bioequivalence to parenteral stability and contamination control strategy CCS.
We hypercharge high-throughput particle-level Raman (MDRS) with deepMorph imaging AI to identify and size tens of thousands of particles per run, reliably down into the sub-micron range. In nasal, inhaled, and topical generics, this gives you the detailed, ISO-traceable evidence you need to support in-vitro bioequivalence and de-risk ANDA/MAA submissions.
For parenteral products, we track proteinaceous particles, silicone oil droplets, glass, fibers, and other extrinsic or intrinsic species across the full lifecycle – from formulation development and device lubrication through long-term stability studies.
Why Choose SizeID.bio?
Why Choose SizeID.bio?
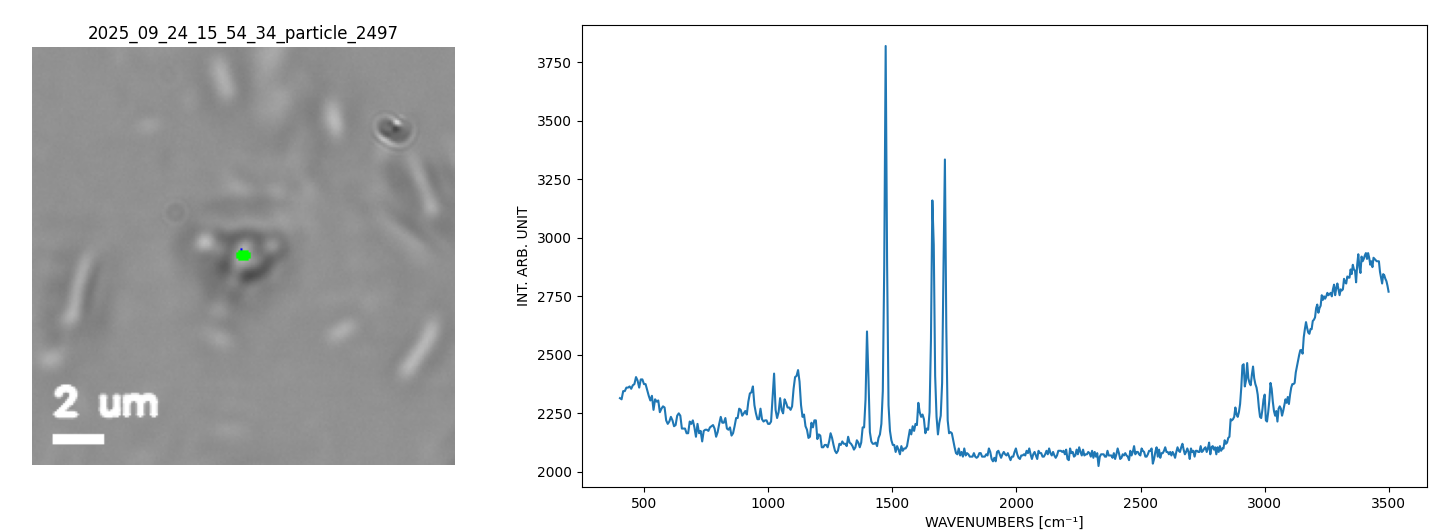
Single API Particle in a Nasal Spray Suspension.
At SizeID.bio, we make the invisible visible — from bioequivalence to stability to contamination control.
🔹Regulators expect robust, reproducible in-vitro evidence.
Different applications need different depth — but they all need clarity, traceability, and confidence.
Our Competitive Edge
🔹 Competitors
• Limited particle counts and slow workflows
• Overnight runs with low statistical depth
• External QC needed to verify signal/noise
• Blind spots in sub-micron ranges
🔹 SizeID.bio
• High-count workflows (10,000+ particles for bioequivalence when required)
• Targeted investigations for CCS & parenterals | focusing on root cause, source tracing, and CAPA
• ~1-6-hour run times instead of overnight
• Built-in signal/noise control | fully aligned with regulatory expectations
• Reliable detection down to 500 nm (Imaging AI + Raman AI)
• ISO 9001 laboratory, validated SOPs, FDA-/EMA-facing reporting
Your Profit:
For bioequivalence, you need statistical power and drug-specific discrimination.
For parenteral stability, you need to track changes over time | proteinaceous vs. silicone oil vs. extrinsic species.
For contamination control, you need to identify the source, link it to your process, and implement effective CAPA.
With SizeID.bio, you get the depth when you need it | and the precision when it matters.